Thiomers
Thiolated Polymers (Thiomers) – Mimicking the Workhorses of Our Body
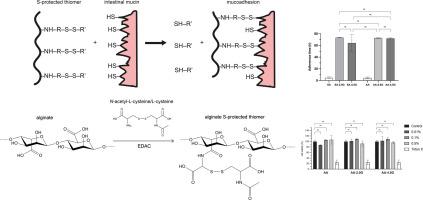
To improve the mucoadhesive properties of polymeric excipients, Andreas Bernkop-Schnürch pioneered the concept of thiolated oligo- and polymers (Thiomers), inspired by one of the most prominent bridging structures in nature — disulfide bonds (1).
By covalently attaching thiol groups to well-established polymers such as cyclodextrins and polysaccharides, a new generation of auxiliary agents for medical and pharmaceutical applications was created. Thiomers mimic proteins—the workhorses of our body—since proteins also contain thiol groups via cysteine residues in their amino acid sequences. Unlike proteins, however, thiomers are structurally less complex, less immunogenic, free from unintended functions, and comparatively easy to manufacture. Furthermore, the degree of thiolation in many proteins is too low for practical application.
The transformative potential of thiomers can best be appreciated by imagining protein chemistry with and without cysteine. Like proteins, thiomers form disulfide bonds with cysteine-rich subdomains of endogenous proteins, and they crosslink through intra- and interchain disulfide bonding. This covalent attachment of thiol groups not only enhances many established polymers but also introduces entirely new properties (2).
Key properties of thiomers include:
- In situ gel formation: Thiomers rapidly crosslink under physiological conditions, creating hydrogels. Thiolated polysaccharides, for example, were shown to increase their viscosity more than 10,000-fold within minutes (3).
- Scaffold formation for tissue engineering: Their disulfide crosslinking enables well-defined polymeric scaffolds that facilitate cell adhesion.
- Bio- and mucoadhesion: By forming disulfide bonds with cysteine-rich subdomains of keratins and mucus glycoproteins, thiomers significantly prolong drug residence time on mucosal surfaces such as the eye or gastrointestinal tract—often by 10- to 100-fold (4, 5).
- Enhanced drug delivery: Through interactions with cysteine-rich membrane proteins, thiomers can improve permeation, enhance cellular uptake, and inhibit efflux pumps, thereby greatly improving the oral bioavailability of many drugs (2, 6).
- Controlled drug release: Drugs containing thiol groups can be attached via disulfide bonds, enabling sustained release in vivo through thiol/disulfide exchange reactions with endogenous thiols (7).
- Metal-binding capacity: Thiomers also show strong affinity for metals such as mercury, gold, and nickel.
Thiomers are also central to the development of thiolated nanoparticles, advancing innovative drug delivery systems (6).
Although it often takes decades for platform technologies in the life sciences to reach their full potential, the benefits of thiomers are already supported by more than 30 human studies and clinical trials. Several thiomer-based products are already on the market, improving the quality of life for millions of patients.
At the 4th Central European Symposium on Pharmaceutical Technology (Vienna, 2001), Bernkop-Schnürch’s group introduced thiolated polysaccharides as novel biopolymers for tissue engineering and regenerative medicine (8). This research gave rise to products such as Blafar (thiolated hyaluronic acid), Glycosil® (thiolated hyaluronan), and Heprasil® (thiolated heparin/thiolated hyaluronic acid).
Thiomers are also being used directly as therapeutic polymers. For example, Lacrimera™ (thiolated chitosan), the first eye drops containing a thiomer, demonstrated significant improvement in dry eye syndrome during clinical trials (9). Marketed under the slogan “Tears for dry eyes”, this innovation received the German Innovation Award (2018). Additionally, thiolated chitosan hydrogels are marketed for dermal use (e.g., Nidiesque®) to treat nickel allergy (10).
Ongoing developments include:
- Injectable self-crosslinkable hydrogels for stem cell therapy (11)
- Vitreous substitutes (12)
- Adhesive wound dressings
- Ocular bandage gels for corneal defects (13) or post-refractive surgery reepithelialization (14)
- Thiolated cyclodextrins as alternatives to aluminum salts for hyperhidrosis
- Eye drops prolonging dexamethasone residence time (15)
- Oral formulations improving the bioavailability of BCS class IV drugs (16)
These advances highlight the broad potential of thiomer technology, which continues to inspire both academia and industry worldwide, leading to a growing number of products and nearly limitless opportunities for pharmaceutical and medical applications.
Latest publications:
References
- Bernkop-Schnürch, A. Mucoadhesive Polymers, Their Use and Their Production Method. Austria Patent AT269105T, 1998; and following patents worldwide.
- Leichner et al. Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature. Adv Drug Deliv Rev. 2019; 151-152:191-221.
- Sakloetsakun et al. In situ gelling properties of chitosan-thioglycolic acid conjugate in the presence of oxidizing agents. Biomaterials. 2009;30(31):6151-7.
- Hornof et al. Mucoadhesive ocular insert based on thiolated poly(acrylic acid): development and in vivo evaluation in humans. J Control Release. 2003, 89(3):419-28.
- Kali et al. Per-thiolated cyclodextrins: Nanosized drug carriers providing a prolonged gastrointestinal residence time. Carbohydr Polym. 2023, 300:120275.
- Hock et al. Thiolated Nanoparticles for Biomedical Applications: Mimicking the Workhorses of Our Body. Adv Sci (Weinh). 2022, 9(1):e2102451.
- Shahnaz et al. Development and in vivo characterization of a novel peptide drug delivery system providing extended plasma half life. J Control Release, 2012, 157(3):375-82.
- Kast et al. Chitosan-thioglycolic acid conjugate: a new scaffold material for tissue engineering? Int J Pharm. 2003, 256 (1–2): 183–189.
- Lorenz et al. Long-term management of dry eye by once-daily use of Chitosan-N-Acetylcysteine (Lacrimera®) eye drops. J Clin Ophthalmol, 2 (2018) 47-54.
- Federer et al. Thiolated Chitosans: A Multi-talented Class of Polymers for Various Applications. Biomacromolecules. 2021, 22(1):24-56.
- Lee et al. Injectable Self-Crosslinkable Thiolated Hyaluronic Acid for Stem Cell Therapy of Atopic Dermatitis. ACS Biomater Sci Eng. 2022 8(4):1613-1622.
- Hurst et al. Long-Term Biocompatibility of a Highly Viscously Thiol-Modified Cross-Linked Hyaluronate as a Novel Vitreous Body Substitute. Front Pharmacol. 2022, 13:817353.
- A Study of the KIO-201 (Ocular Bandage Gel) for Improving Persistent Corneal Epithelial Defects (2022).
- Durrie et al. Ability of a new crosslinked polymer ocular bandage gel to accelerate reepithelialization after photorefractive keratectomy. J Cataract Refract Surg. (2018) 44(3):369-375.
- Grassiri et al. Int J Mol Sci. 2022 Feb 26;23(5):2612.
- Asim et al. Per-6-Thiolated Cyclodextrins: A Novel Type of Permeation Enhancing Excipients for BCS Class IV Drugs. ACS Appl Mater Interfaces. 2020 12(7):7942-7950.