Thiolated Cyclodextrins as Drug Delivery Systems
Optimizing drug carriers for patients needs
Cyclodextrins (CD, or cycloamylose) are cyclic α-(1–4)-linked oligosaccharides consisting of α-d-glucopyranose units. These compounds are widely used because they are (sub)nanosize, non-toxic, biodegradable, and readily available materials that can be easily functionalized. Among others, the biggest advantage of CDs is that they complex and solubilize various hydrophobic substances, including drugs and polymers. The hydrophobic cavity of CDs binds suitable organic molecules through hydrophobic interactions and/or hydrogen bonds, while the hydrophilic outer shell maintains water solubility.[1] Although CDs are already widely used in drug delivery, their application is limited by their relatively short residence time in the gastrointestinal tract and poor cellular uptake, due to the lack of interactions with the mucus layer and cellular membranes, respectively.
Therefore, we have thiolated CDs through various methods, resulting in a wide range of modification levels and having versatile physical and chemical properties.[2]
- Oxidative opening of some glucopyranose repeats, followed by reductive amination, is used to modify CDs with cysteamine or cysteine moieties, resulting in high aqueous solubility, but a significantly altered structure. The degree of modification also remained low with this multistep reaction.[3-7]
- Direct hydroxyl-to-thiol conversion of CDs via halogen or isothiouronium intermediates results in a slightly higher degree of substitution but decreases water solubility.[8-10]
- The recently developed reaction involving phosphorus pentasulfide (P₂S₅) is a single-step process that results in high degrees of thiolation. This simple reaction was the first to achieve per-thiolation of a CD.[11]
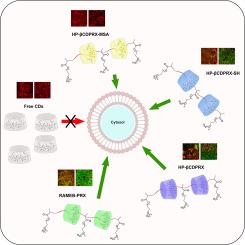
Thiolated CDs are used as mucoadhesive drug delivery systems, greatly prolonging the gastrointestinal residence time, and also as permeation enhancers. These thiomers improved the cellular uptake of model drugs and inhibited P-glycoprotein-mediated efflux. The main drawback of thiolated CDs, their oxidative sensitivity to form disulfide bonds before reaching the deep firm mucus layers, was addressed by protecting the free thiols. This S-protection allows for deeper diffusion, stronger mucoadhesion, and further enhanced cellular uptake.[12-14]
Recently, CDs have been used not as freely dissolved excipients, but as supramolecular polymeric assemblies threaded onto polymer chains. These, so-called polyrotaxanes, demonstrate enhanced cellular uptake and CD release in the cytosol. Their application in treating lysosomal storage diseases is confirmed and still under detailed investigation.[15-17]
Latest publications:
References
- G. Kali, S. Haddadzadegan, and A. Bernkop-Schnürch, “Cyclodextrins and derivatives in drug delivery: New developments, relevant clinical trials, and advanced products,” Carbohydrate Polymers, vol. 324, p. 121500, 2024/01/15/ 2024, doi: https://doi.org/10.1016/j.carbpol.2023.121500.
- G. Kali, P. Knoll, and A. Bernkop-Schnürch, “Emerging technologies to increase gastrointestinal transit times of drug delivery systems,” Journal of Controlled Release, vol. 346, pp. 289-299, 2022/06/01/ 2022, doi: https://doi.org/10.1016/j.jconrel.2022.04.016.
- M. Ijaz et al., “Development of pre-activated α-cyclodextrin as a mucoadhesive excipient for intra-vesical drug delivery,” International Journal of Pharmaceutics, vol. 534, no. 1, pp. 339-347, 2017/12/20/ 2017, doi: https://doi.org/10.1016/j.ijpharm.2017.10.054.
- M. H. Asim, A. Jalil, I. Shahzadi, M. Khan, B. Matuszczak, and A. Bernkop-Schnürch, “Mucoadhesive S-protected thiolated cyclodextrin-iodine complexes: a promising strategy to prolong mucosal residence time of iodine,” (in eng), Future Microbiol, vol. 14, pp. 411-424, 03 2019, doi: 10.2217/fmb-2018-0288.
- M. Ijaz et al., “Synthesis and characterization of thiolated beta-cyclodextrin as a novel mucoadhesive excipient for intra-oral drug delivery,” Carbohydr Polym, vol. 132, pp. 187-195, 2015, doi: 10.1016/j.carbpol.2015.06.073 Epub 2015 Jun 25.
- M. Ijaz, M. Ahmad, N. Akhtar, F. Laffleur, and A. Bernkop-Schnürch, “Thiolated alpha-Cyclodextrin: The Invisible Choice to Prolong Ocular Drug Residence Time,” (in eng), J Pharm Sci, vol. 105, no. 9, pp. 2848-2854, 2016, doi: 10.1016/j.xphs.2016.04.021 Epub 2016 May 25.
- M. Ijaz, J. A. Griessinger, A. Mahmood, F. Laffleur, and A. Bernkop-Schnürch, “Thiolated Cyclodextrin: Development of a Mucoadhesive Vaginal Delivery System for Acyclovir,” Journal of Pharmaceutical Sciences, vol. 105, no. 5, pp. 1714-1720, 2016/05/01/ 2016, doi: https://doi.org/10.1016/j.xphs.2016.03.009.
- M. Asim et al., “S-protected thiolated cyclodextrins as mucoadhesive oligomers for drug delivery,” Journal of Colloid and Interface Science, vol. 531, 07/01 2018, doi: 10.1016/j.jcis.2018.07.062.
- M. H. Asim, I. Nazir, A. Jalil, F. Laffleur, B. Matuszczak, and A. Bernkop-Schnürch, “Per-6-Thiolated Cyclodextrins: A Novel Type of Permeation Enhancing Excipients for BCS Class IV Drugs,” (in eng), ACS Appl Mater Interfaces, vol. 12, no. 7, pp. 7942-7950, Feb 2020, doi: 10.1021/acsami.9b21335.
- M. Hussain Asim, I. Nazir, A. Jalil, B. Matuszczak, and A. Bernkop-Schnürch, “Tetradeca-thiolated cyclodextrins: Highly mucoadhesive and in-situ gelling oligomers with prolonged mucosal adhesion,” International Journal of Pharmaceutics, vol. 577, p. 119040, 2020/03/15/ 2020, doi: https://doi.org/10.1016/j.ijpharm.2020.119040.
- G. Kali, S. Haddadzadegan, F. Laffleur, and A. Bernkop-Schnürch, “Per-thiolated cyclodextrins: Nanosized drug carriers providing a prolonged gastrointestinal residence time,” Carbohydrate Polymers, p. 120275, 2022.
- G. Kali et al., “Enhanced Mucoadhesion of Thiolated β-Cyclodextrin by S-Protection with 2-Mercaptoethanesulfonic Acid,” ACS Omega, vol. 9, no. 5, pp. 5819-5828, 2024/02/06 2024, doi: 10.1021/acsomega.3c08836.
- S. Haddadzadegan et al., “Cyclodextrin-mediated enhancement of gastrointestinal drug delivery: Unveiling mucoadhesive and mucopenetrating synergy,” ed. Submitted Manuscript, 2024.
- S. Haddadzadegan, P. Knoll, R. Wibel, G. Kali, and A. Bernkop-Schnürch, “Three generations of thiolated cyclodextrins: A direct comparison of their mucus permeating and mucoadhesive properties,” Acta Biomaterialia, vol. 167, pp. 309-320, 2023.
- G. Kali et al., “Polycaprolactone/α-cyclodextrin polyrotaxanes with cellular uptake enhancing properties,” (in eng), J Mater Chem B, vol. 13, no. 10, pp. 3471-3482, Mar 5 2025, doi: 10.1039/d4tb02451f.
- G. Kali et al., “Disulfide Stoppered Polyrotaxanes with Enhanced Cellular Uptake and Intracellular Cyclodextrin Release,” vol. 8, ed. Carbohydrate Polymer Technologies and Applications, 2024, p. 100586.
- D. Gintsburg et al., “Cyclodextrin-based polyrotaxanes: Cellular uptake enhancement effect of different functionality types,” Carbohydrate Polymer Technologies and Applications, vol. 11, p. 100981, 2025/09/01/ 2025, doi: https://doi.org/10.1016/j.carpta.2025.100981.